Week 3: Oct 27 – Oct 31, 2025
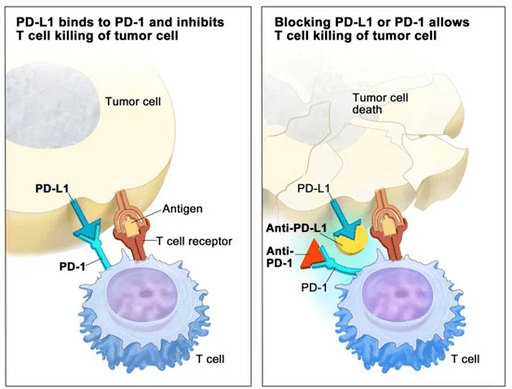
💉 Merck Clinches Another Approval for Its PD-1 Inhibitor Keytruda in Head and Neck Cancer
🧾 What happened?
Immunotherapy remains one of the most promising advances in cancer therapy over the past decade.
Prototypical in this class is Merck’s blockbuster PD-1 inhibitor, Keytruda (pembrolizumab), which acts to “release the brakes” on the immune system.
On Wednesday, Merck announced that the European Commission approved Keytruda for PD-L1–positive head and neck cancer, following the FDA’s approval for the same indication earlier this year in June.
🔬 What was found?
The decision was based on results from the Phase III KEYNOTE 689 trial, a randomized, active-controlled, open-label study evaluating pembrolizumab in locally advanced head and neck squamous cell carcinoma (HNSCC).
The drug was administered both before and after surgery, in combination with standard of care radiotherapy (with or without cisplatin chemotherapy).
Patients receiving the Keytruda regimen achieved a median event-free survival of 59.7 months compared with 29.6 months in the control group and showed a 30 percent reduction in the risk of recurrence, progression, or death.
💡 Why does it matter?
Merck’s ongoing strategy of expanding combination approvals for Keytruda is clearly paying off.
These results reinforce how PD-1/PD-L1 checkpoint inhibition continues to reshape oncology, improving survival across multiple tumor types — including melanoma, non-small-cell lung cancer (NSCLC), renal cell carcinoma (RCC), HNSCC, and urothelial carcinoma — as well as tissue-agnostic indications such as MSI-H tumors.
Still, immunotherapy is typically effective only in immunologically “hot” tumors (those with high PD-L1 expression), and the development of resistance remains a major challenge.
Time will tell whether Merck and other companies can continue expanding checkpoint inhibitors across additional cancer types.
🔗 Sources
- Official Announcement from Merck
- Phase III KEYNOTE 689 trial, ClinicalTrials.gov
- Image credit: Wikimedia Commons, “Scheme how checkpoint blockers work”
💉 Could the COVID-19 Vaccine Help Fight Cancer Too?
🧾 What happened?
In addition to halting the pandemic and saving millions of lives, the COVID-19 mRNA vaccines may also have an unexpected benefit in cancer treatment.
An exciting new study led by researchers from MD Anderson Cancer Center and the University of Florida found that mRNA vaccination significantly increased survival in lung and skin cancer patients undergoing immunotherapy.
🔬 What was found?
The study, published this month in Nature, discovered that receiving a SARS-CoV-2 mRNA vaccine within 100 days of starting immune checkpoint inhibitor therapy was associated with substantial improvements in overall survival (OS) in patients with non-small-cell lung cancer (NSCLC) and melanoma.
The findings were based on analysis of more than 1,000 patient records from MD Anderson.
While the results are preliminary, the researchers are now designing a randomized clinical trial to confirm these findings.
💡 So what?
Immunotherapy remains one of the most exciting advances in cancer treatment in recent years, including checkpoint inhibitors (PD-1/PD-L1 inhibitors) and CAR-T therapy.
However, a major limitation of checkpoint inhibitors is that the tumor must be immunologically active, often characterized by high PD-L1 expression.
These new results suggest the possibility of a universal cancer vaccine that could prime the immune system against multiple tumor types.
If confirmed, this research could open entirely new avenues for immunotherapy development and cancer prevention.